
There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.
Atomic mass of boron plus#
Neutron number plus atomic number equals atomic mass number: N+ZA. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Please note that the elements do not show their natural relation towards each other as in the Periodic system. Mass numbers of typical isotopes of Boron are 10 11. The unity for atomic mass is gram per mol. The lightest chemical element is Hydrogen and the heaviest is Hassium. The chemical elements ofįor chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). If 10B has a mass of 10.013 u and 11B has a mass of 11.006 u, what are the percent. 3 Problem Which of the following choices has the greatest atomic mass A Element A (0.283 kg) B Element B (3.20 x 10 24 amu) C Element C (0. There are only two naturally occurring isotopes of Boron, 10B and 11B. This list contains the 118 elements of chemistry. 2m Mark as completed Was this helpful 2 example Atomic Mass Example 1 2m Mark as completed Was this helpful On the Periodic Table, the atomic mass is represented by the number with decimal places. For example, the element boron is composed of two isotopes: About 19.9 of all boron atoms are 10 B with a mass of 10.0129 amu, and the remaining 80.1 are 11 B with a mass of 11.0093 amu. Separation and Concentration Purification RequestĬhemical elements listed by atomic mass The elements of the periodic table sorted by atomic massĬlick on any element's name for further information on chemical properties, environmental data or health effects.Plant Inspection & Process Optimalisation.Calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries. Additional technical, research and safety (MSDS) information is available as is a Reference Calculator for converting relevant units of measurement. The actual atomic mass of boron can vary from 10.807 to 10.819, depending on whether the mineral source is from Turkey or the United States. Typical and custom packaging is available. American Elements produces to many standard grades when applicable, including Mil Spec (military grade) ACS, Reagent and Technical Grade Food, Agricultural and Pharmaceutical Grade Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Boron Metal 10 isotopic material is generally immediately available. One atomic mass unit is equal to 1.66 x 10 -24 grams. The unit of measure for mass is the atomic mass unit (amu).
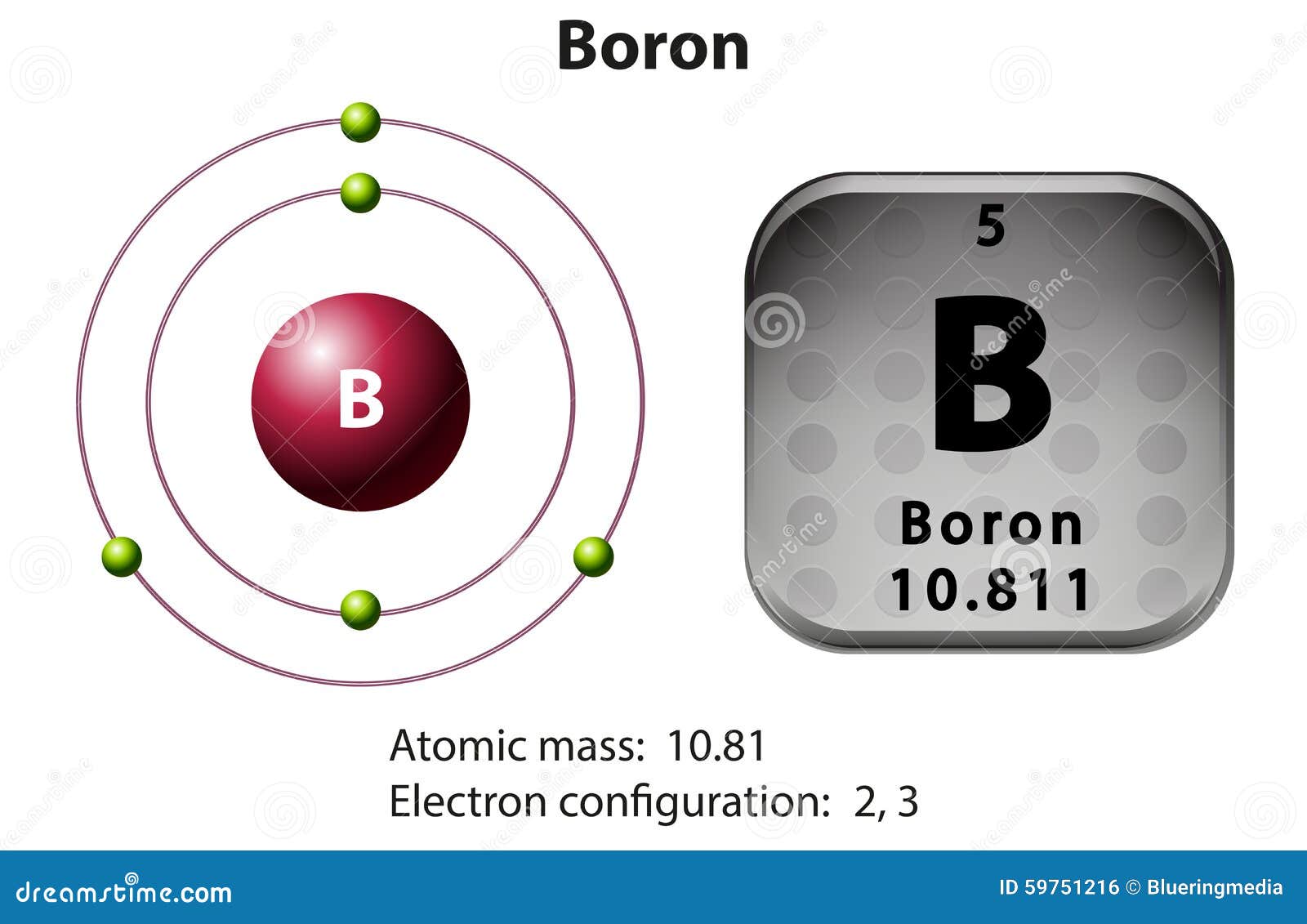
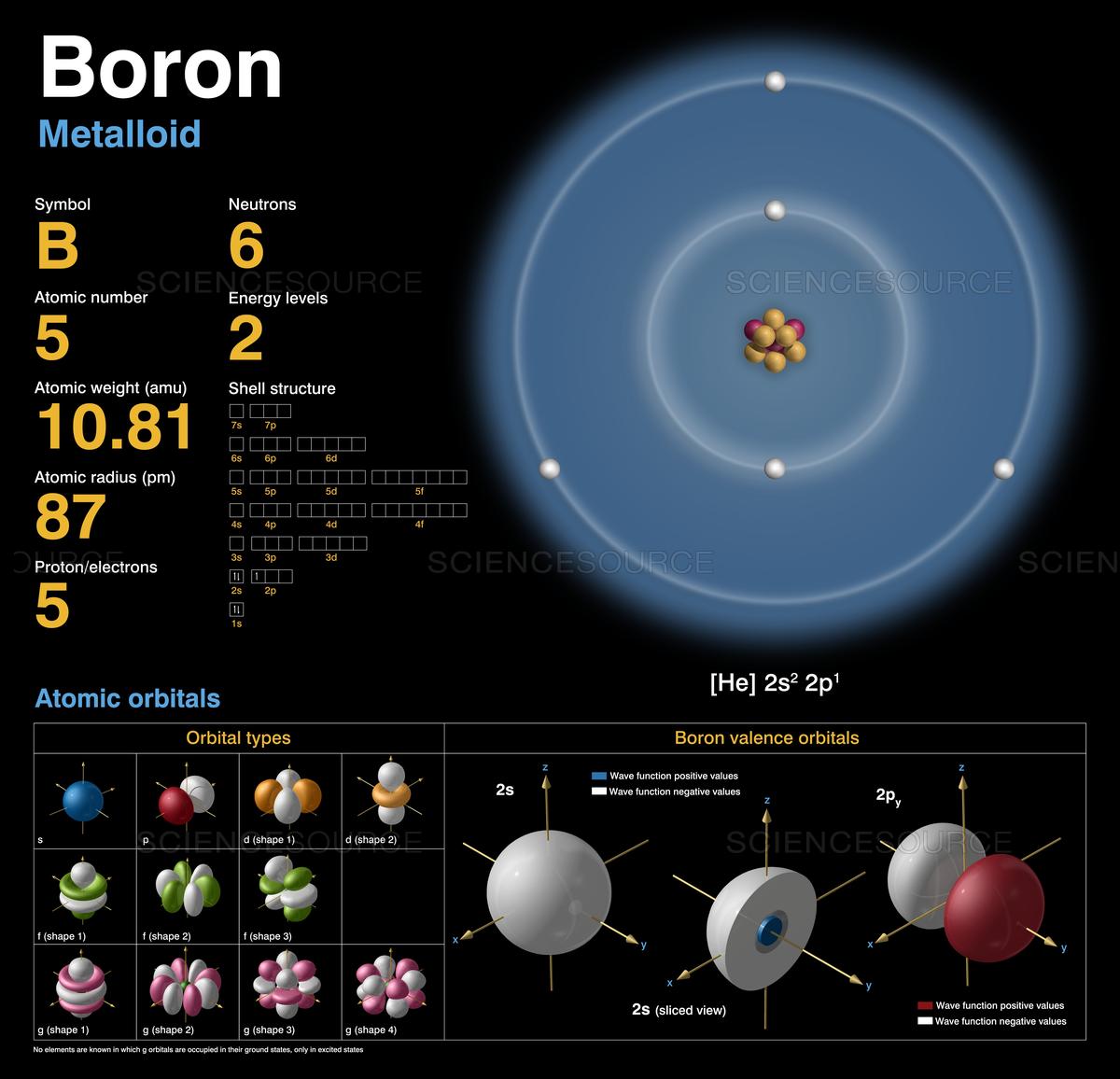

Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance. For thin film applications it is available as rod, pellets, pieces, granules and sputtering targets and as either an ingot or powder. Note that each element may contain more isotopes.
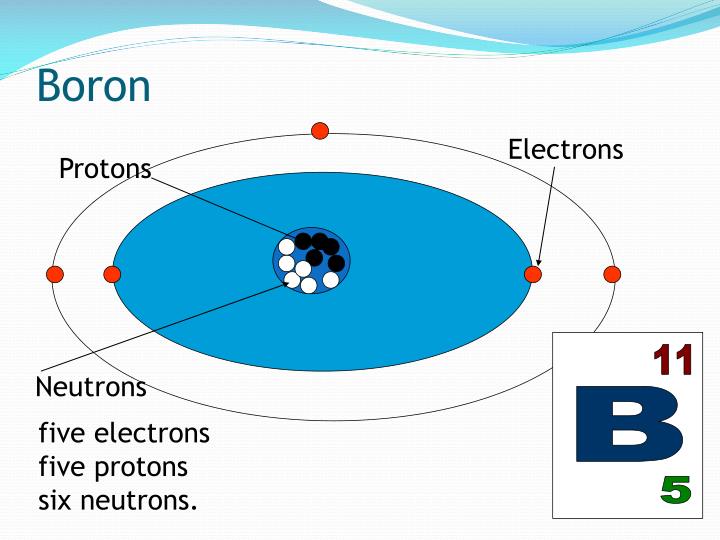
Boron Metal is also available in ultra high purity and as nanoparticles. An element is made up of atoms with the same atomic number, which means that each atom in the component has the. Boron Metal 10 additionally has special application in extrinsic food labeling used to determine boron metabolism. Boron 10 Metal is one of over 250 stable Metallic isotopes produced by American Elements for biological and biomedical labeling, as target materials and other applications. It is both naturally occurring and a produced by fission.


 0 kommentar(er)
0 kommentar(er)
